

* Offer not applicable to German market.
SMARTMSG
- Broadest range of applications – wide selection of treatment accessories
- Simple and intuitive operation
- Extensive database of procedures with the possibility of their modification and assignment to the patient
- Greater precision of procedures with limited invasiveness for tissues
- Quick return to daily activities
- Pain reduction
- No need for hospitalization
- Lowest operating costs
A brief history of laser therapy
The history of laser application in medicine began with surgical lasers, formerly referred to as laser knives.
American scientist Theodore Maiman was the first to successfully build a laser. This coherent and monochromatic laser light beam shone for the first time in 1960 and began a promising era, giving rise to all modern solid-state lasers.
In October 1961, Elias Snitzer of American Optical Co. reported on the first neodymium laser surgery.
In December 1961, the first medical laser procedure was performed by Doctor Charles Campbell of the Institute of Ophthalmology at Columbia-Presbyterian Medical Center and Charles Koester of American Optical Co. at Columbia-Presbyterian Hospital in Manhattan. An American optical ruby laser was used to destroy a retinal tumor.
In October 1962, Nick Holonyak, a consultant at General Electric Co.’s lab in Syracuse, published his work on the ‘visible red’ GaAsP laser diode – a compact and efficient source of visible coherent light.
In 1963, the first Polish HE-NE laser was put into operation – created at the Military University of Technology and generating radiation of the 1.15 µm wavelength.
In 1964, the first diode-pumped semiconductor laser was built.
In 1972, the widespread use of lasers in world medicine began. The advent of optical fibers was the main reason for looking for new laser applications in medicine. Optical fibers made it possible to insert the laser beam into body cavities and cavernous tissues.
Lasers in proctology were first demonstrated in 1989. It involved a surgical excision of hemorrhoids. In 1990, Masson described cases of patients who underwent CO2 laser hemorrhoidectomy in an outpatient setting with good results.
Each laser can be upgraded with a 635nm handpiece, ideal for biostimulation and wound care, as well as a 405nm handpiece specifically designed for diagnostics of infected areas and bacteria detection.
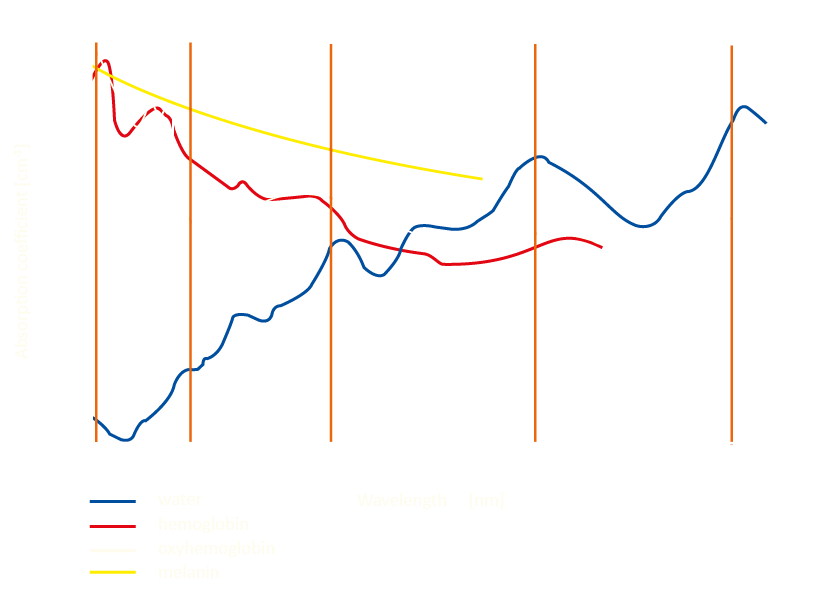
Why is there no single wavelength?
A novelty among the medical products manufactured by Lasotronix is SMARTMSG 980/1470nm – a two-wave universal surgical laser, which in addition to phlebology and proctology is widely used in dermatologic surgery, otolaryngology, and gynecology.
Furthermore, diode lasers from the SMART family can be extended with an additional wavelength of 635nm, which – owing to biomodulation effects – has an incredibly positive impact on human blood morphotic elements. Treatment with this wave significantly improves the process of soft tissue regeneration, rebuilds blood vessels, reduces post-operative pain and swelling, stimulates blood and lymph flow, and strengthens local immunity. The use of the 635nm laser is especially recommended for patients suffering from inflammation, hard-to-heal wounds or ulcers, and after any surgery to accelerate healing, reduce pain and swelling.
For minimally invasive intravenous laser ablation (EVLT) Lasotronix offers diode lasers that emit radiation at different wavelengths, i.e., 980nm, 1470nm or 1940nm, with the possibility of their further expansion with additional light sources.
As can be seen from the graph, the absorption coefficient for the 980nm radiation is higher for hemoglobin than for water. In case of 1470nm, especially 1940nm, the opposite is true, and water absorbs light radiation many times better than hemoglobin. Both of these chromatophores are components of human tissues, including blood and vessel walls. Lasers with a wavelength of 980nm have been applied extensively for more than 20 years, and the average power used in treatments is 10-12W. At 1470nm, lower power values of 6-8W are needed, and for 1940nm, 4-6W are enough to perform an effective operation. Reducing the amount of power needed makes these lasers much safer and predictable tools for the physician, while providing patients with a faster and more comfortable recovery.
Specifications:
- Laser type: diode
- Output power: up to 15 W for 980nm, 1470nm
- Output power: 7 W for 1940nm
- Output power: 0.5W for 405nm, 635nm,
- Screen: color, touch
- Fiber diameter: 200μm, 320μm, 400μm, 600μm
- Weight: 2.5 kg

HEMORRHOIDS WITH CONICAL FIBER SET
- Proctology handpiece (including adapter luer slip (LS) internal diameter 2.5mm for proctology) (Part no.: 170042)
- Conical Fiber (600µm fiber core, 3m long, standard SMA 905 connector with knurl nut, disposable (Part no.: 250026)
- Proctology cannula 14Gx6cm (Part no.: 170032)

HEMORRHOIDS, SOFT TISSUE CUTTING AND COAGULATION SET
- Handpiece for microsurgery applications (Part no.: 170027.02)
- Proctology cannula L50R110 curved (length 50 mm) (reusable) (Part no.: 170040.01)
- Bare optical fiber (600μm fiber core, 3m long, SMA905 connector/ open-ended, reusable – up to 10 sterilizations) (Part no.: 250019)

EVLT WITH DISPOSABLE BARE FIBER AND CATHETER SET
- Bare optical fiber (600μm fiber core, 3m long, SMA905 connector/ open-ended, disposable) (Part no.: 250017)
- EVLT Catheter 14G for bare fiber, 70cm long, Seldinger Puncture needle18 G – 7 cm, Dilator 7 F – 10 cm, Guide wire 0.035’’, 150cm J-tip (Part no.: 250020)
- Lock Adapter for EVLT Catheter (Part no.: 250022)

EVLT WITH FUSED RADIAL EMISSION FIBER AND CATHETER SET
- Optical fiber with radial energy emission SLIM (600μm fiber core, 2.6m long, SMA905 connector/ radial tip, disposable) (Part no.: 250024)
- Catheter set for Fiber with Radial Energy Emission 6F (sheath introducer, dilator, guidewire, puncture needle) (Part no.: 250025)
* Offer not applicable to German market.

BIOMODULATION SET
- Therapeutic laser handpiece 635nm (Part no.: 170043.01)
- Connection cable (between diode laser housing and therapeutic handpiece) (Part no.: 170031)
- Therapeutic tip for biostimulation (lenticular) ø14mm for 635nm/405nm (Part no.: 170020)
- Safety glasses for 635nm (Part no.: 260001)

DIAGNOSTICS SET
- Therapeutic laser handpiece 405nm (Part no.: 170043.02)
- Therapeutic tip for biostimulation (lenticular) ø14mm for 635nm/405nm (Part no.: 170020)
- Safety glasses for 405nm (Part no.: 260006)
Leave us your e-mail address or telephone number. We will contact you.
Pursuant to Article 13 of the General Data Protection Regulation of 27 April 2016 (Official Journal of the European Union EU L 119 of 04/05/2016) hereinafter referred to as “GDPR”, I hereby inform that:
- the Controller of your personal data is Lasotronix Sp. z o.o. with its registered office at ul. Elektroniczna 2A , 05-500 Piaseczno, Poland
- you can contact the Controller via email at biuro@lasotronix.pl
- your personal data will be processed in order to establish contact on the basis of Article 6(1)(f) of the GDPR as a legitimate interest of the Controller and for marketing purposes on the basis of Article 6(1)(a) of the GDPR – consent of the data subject
- the recipients of your personal data will be only entities authorized to obtain personal data under the law, website management entities and mass mailing service providers
- your personal data will be stored until you withdraw your consent; you can withdraw your consent at any time
- you have the right to request from the Controller access to your personal data, the right to rectify, erase or restrict the processing
- you have the right to lodge a complaint with the supervisory authority
- providing personal data is voluntary; however, failure to provide such data may result in the inability to establish contact and receive marketing offers or to participate in surveys

